Biopharma and Life Sciences
The Benefits of Process Architecture in Facility Design

Biopharma and Life Sciences
The Benefits of Process Architecture in Facility Design
The rapidly evolving demands of pharmaceutical manufacturing are driving engineering firms to adopt more flexible, integrated approaches to facility design. Donato de Vivo, Manager Architectural, discusses.
Rethinking facility design: The benefits of process architecture
The rapidly evolving demands of pharmaceutical manufacturing are driving engineering firms to adopt more flexible, integrated approaches to facility design. At the heart of this shift is the emerging role of the process architect, a multidisciplinary professional who bridges engineering, sustainability, and the shift toward modular lab environments. As facility design grows more complex, with increasing regulatory, environmental, and technological demands, the process architect’s role has become essential. Process architects are vital for firms aiming to future-proof pharmaceutical facility designs and achieve compliance with regulatory and internal standards. This case study covers how process architects help facilitate sustainability, flexibility, compliance, innovation, and safety in designing modern pharmaceutical process facilities.
Enhancing sustainability
Sustainability is a top priority for many stakeholders across process industries, including pharmaceuticals. Companies are increasingly aligning with international sustainability goals, such as the EU Green Deal, which targets net-zero greenhouse gas emissions by 2050. To meet these long-term objectives, sustainable practices must be embedded into the foundation of facility design. This includes strategies to conserve water, reduce energy use, and limit emissions, ultimately minimizing the facility’s impact on local communities and the global environment.
Promoting sustainability is a key responsibility of process architects, who design facilities that align with both client-specific goals and broader global initiatives. To achieve this, they apply a range of strategies and tools, including:
Value Engineering and Cost Reduction: Optimizes material and energy usage while minimizing environmental and financial waste throughout the project lifecycle.
Box-in-Box Approach: Isolates critical environments to reduce energy loads, HVAC demand, and long-term maintenance costs.
Closed Processes and Ballroom Layouts: Minimize cross-contamination and reduce the need for excessive cleanroom classification, leading to smaller, more efficient spaces.
Using strategies like these, process architects help facilities align with broader industry trends toward green manufacturing and building the “Facility of the Future.”
Improving flexibility
Adaptability is a cornerstone of modern pharmaceutical production facilities. The ability to scale processes up or down, without running out of space or wasting resources on underused lab areas, is key to reducing costs and operational inefficiencies. Flexible spaces also help minimize downtime by avoiding major construction or reorganization when production needs change.
Process architects are crucial in ensuring flexibility from the earliest design phases. What may seem like minor decisions, such as corridor widths or ceiling heights, can have a major impact later. For instance, if a facility plans to use a 600/900-liter bioreactor, a process architect ensures not only that it fits in the production room, but that the entire facility can accommodate its delivery, installation, maintenance, and transfer to other areas, ensuring doorways, corridors, and overhead clearances are sufficient. Without this foresight, costly delays and retrofits are inevitable.
Process architects also design spaces with temporary or mobile partitions to support process segregation, and scalable locker rooms and changing areas that can adapt to fluctuating staff numbers or demographics.
Supporting regulatory compliance
Compliance spans multiple dimensions, from pharmaceutical regulations such as FDA, EMA, and WHO standards, to region-specific labor and environmental laws, as well as internal company policies (Fig. 1). Process architects bring a deep working understanding of regulatory requirements, helping clients not only meet but maintain compliance throughout the operational lifecycle, making regulatory adherence a seamless part of everyday processes.
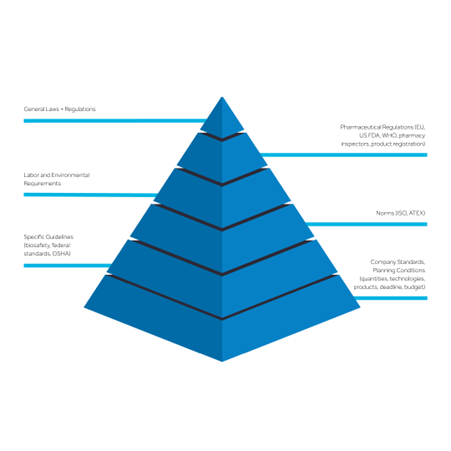
By planning for these requirements early, process architects establish proper material and personnel flows, zoning, and spatial configurations that prepare facilities for regulatory audits with minimal disruption.
Driving innovation
Process architects enable innovation by designing facilities that can seamlessly integrate emerging technologies, such as ExyCell® modules, into existing manufacturing modules. Their approach to flexibility allows companies to adapt quickly and cost-effectively, whether that means reconfiguring layouts, scaling processes, or trialing new workflows without major capital investment.
This adaptability is crucial for implementing non-standard process flows required by new formulations or therapy modes. Modular systems make it easier to introduce new technologies into existing workflows, allowing facilities to adopt innovations earlier and stay ahead of the curve.
With deep multidisciplinary experience, process architects are perfectly positioned to identify opportunities for innovation, whether in spatial design, equipment layout, or ergonomics. Their early involvement in a project promotes smarter decisions from the outset, enabling spatial optimization and system integration.
Reducing risk and improving safety
Pharmaceutical production carries risks on multiple fronts: risks to personnel safety, risks to product quality through contamination, and risks of non-compliance with stringent regulatory standards. Process architects play a critical role in mitigating all of these by taking a holistic and proactive approach to safety. By incorporating health, safety, and environmental (HSE) considerations early in the design phase, they help ensure that safety is not an afterthought but a foundational element of facility planning.
Contamination control
Protecting products, processes, and personnel starts with robust contamination control strategies. These include carefully planning material and personnel flows, implementing proper zoning, and designing airlock systems to prevent cross-contamination between sterile and non-sterile zones. In addition to these considerations, process architects ensure that gowning areas are accessible, adaptable, and able to accommodate evolving safety protocols or changes in process requirements over time.
Conclusion
Process architects bridge the critical gap between vision and execution in life sciences facilities, ensuring that design meets current needs and anticipates future demands. As strategic partners, they bring together technical, regulatory, and operational elements to deliver compliant, flexible facilities built for innovation. Their ability to align spatial design with complex process requirements makes them indispensable in creating future-proof environments.
This article has only scratched the surface of the enormous and enduring value that involving a process architect from the very beginning of facility design can provide.
Our full white paper explores the topics discussed above in greater depth and includes practical guidance, including a process architecture knowledge checklist, to help you choose the right partner.
Contact an expert at Exyte to learn how a process architect can help future-proof your production facility, reduce risks, and ensure you're set up for success, both now and in the years ahead.